PHARMACO-EEG
Home » Solutions / Preclinical EEG Services / Preclinical Assays / Pharmaco-EEG
Pharmaco-EEG Studies in the Preclinic Are Key to Understand and Prepare Clinical Trial
Currently, one of the most important pain points in drug discovery lies in the ability to predict the outcomes of clinical trials. This predictability centers on the design of preclinical studies and the endpoints selected.
Since the modulation of neurotransmitters will induce specific changes in extracellular field potentials, EEG and more specifically pharmaco-EEG (qEEG) appears to be an important step in the preclinical drug development journey. Although these types of studies appear to be essential, some problems persist in this area such as the reproducibility of studies depending on multiple factors (animal background, electrode implantation sites, computations, etc.), as well as the environmental conditions. Our strong expertise and efforts towards standardizing this technique are, therefore, decisive.
Bridging the Preclinical to Clinical Gap Thanks to qEEG
Through its endeavors to promote standardized and clinically-relevant studies from the preclinical stage, SynapCell has focused on EEG technology and the different endpoints it can produce. Our pharmaco-EEG data have been generated with the same electrode implantations and the same recording conditions to promote reproducibility and increase translatability to humans and the clinic. Coupled with the expertise of our scientists, this standardization represents an essential passage before proceeding to the IND and the clinic.
Preparing for the Potential Effect in Phase 1 EEG Studies
We use EEG’s highly translational power to predict clinical outcomes.
The cross-species consistency of EEG helps predict Phase 1 trial results, and more importantly guides where to look. Compound-induced spectral changes that are observed preclinically are likely translate to humans, helping to guide clinical focus and inform go/no-go decisions in drug development.
Confirming Target Engagement by the Compound in Naïve Animals
We use a crossover design in most studies, particularly in acute conditions. Crossover design allows animals to serve as their own controls across multiple conditions. Increasing the number of conditions also allows us to test various doses of the same compound. EEG detects subtle spectral changes, providing precise information on target engagement and compound effects, including timing, duration, and effective dose.
Different Levels of Post-hoc Analysis to be Implemented
Our standard pharmaco-EEG setup includes multiple depth and surface EEG electrodes, plus EMG (Electromyography) electrodes at the base of the neck.
This allows us to gather different types of data for analysis that can be added to and implemented weeks after the end of the experiments. One of the additional powers of EEG recording is that the raw data can be exploited multiple times to perform different analysis, without time limitations.
A Comprehensive Range of qEEG Solutions
SynapCell offers two distinct qEEG solutions to meet diverse evaluation needs depending on the readouts required by our Sponsors when testing their compounds.
SynapCell's qEEG Solution
Classical profiling of compounds with varied mechanism of actions:
this quantitative analysis of EEG (qEEG) signals can be used to assess brain activity and compound efficacy, providing insights into classical spectral bands.
ADVANCED qEEG FOR SLEEP & VIGILANCE STATES
This comprehensive option includes the standard qEEG analysis plus additional readouts for sleep and three vigilance states, offering a more in-depth evaluation of the compound’s effects on sleep-wake cycles and various states of vigilance.
Key Assets
of Pharmaco-EEG
A direct comparison of several therapeutic agents in consistent and standardized conditions (same electrode placement, same timings of injections, etc.) bringing the translational validity of preclinical Pharmaco-EEG studies to a new level.
EEG is a highly conserved feature across species and particularly across mammals. Consequently, the scientific community considers this technology is one of the most translational tools out there.
We offer the possibility of long-duration EEG recordings to fully describe the pharmacodynamics of compounds, but also to study the impact of the drug candidate on sleep stages, sleep cycles, and sleep architecture.
Drug developers often face challenges due to insufficient PK profiles in humans. Pharmaco-EEG studies are a powerful tool to pre-empt these challenges by documenting the PK/PD properties of your novel innovative drug thanks to sufficiently long-duration studies to observe a “back to baseline” trend.
Drug Discovery Assays with the Pharmaco-EEG Method
Discriminate the candidates that best suit your expectations that have the best chance of succeeding in the clinic, based on their EEG spectra.
Fine-tune the target-engagement settings of your compound by testing different doses of compounds to see when changes in the parameters measured are induced.
Evaluate a drug’s long-term effects and safety when administered repeatedly over an extended period. qEEG can assess efficacy, toxicity, and potential side effects during prolonged use. Of course, we also study tachyphylaxis in these designs by following, over the different days of dosing, any drop-off in efficacy following compound administration.
Study how drugs interact with/affect the body, and examine the biological and physiological effects of your compounds, their mechanisms of action, and the relationship between drug concentration and EEG modulation.
Evaluate the impact of compounds on sleep and vigilance states using polysomnography to analyze sleep architecture as well as advanced qEEG.
POSTER
Decoding Therapeutic Signatures: The Contribution of Pharmaco-EEG when Profiling Compounds with Varied Mechanisms of Action
This poster reports some of the analyses performed in-house. The main analyses performed were FFT and polar plots, with the goal of defining the potential specific signature associated with a therapeutic class of compounds on a higher level. SynapCell is continuously adding to this dataset and growing its pipeline by profiling new compounds. Get in touch if you have a specific compound/mechanism of action you would like to see!
DOWNLOAD
POSTER

Powered by Cue®, SynapCell's Predictive In Vivo EEG Platform
SynapCell’s pharmaco-EEG recordings are processed on Cue®, our innovative translational in vivo EEG platform, which is designed to predict the in-human efficacy of your drug candidates during the preclinical step. Cue® is the result of decades of R&D, combining SynapCell’s know-how, expertise and scientific excellence in the fields of brain surgery and EEG signal recording, processing, and analysis.
Using Cue®, we transform preclinical data into actionable insights, offering end-to-end support for informed decision-making in CNS drug discovery.
THE SCIENCE CORNER
Recording Parameters & Electrode Implantations
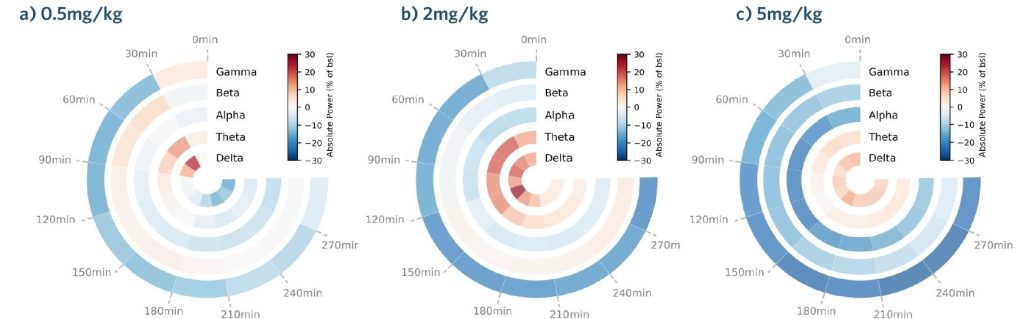
SynapCell’s recordings are generated thanks to EEG and EMG electrodes, across distinct brain regions in C57BL/6J mice. As the standardization of electrode implantations is crucial, we use stereotaxic surgery to be as reproducible as possible, with precise coordinates for each structures. In addition, we implant three types of electrodes: depth electrodes (to record LFP and more specific structures such as the hippocampus), surface electrodes implanted on the surface of the skull (to record broader signals over the motor cortex, for example), and EMG electrodes in the nuchal muscles (to capture muscle activity and identify sleep stages). Since EEG recordings generate large volumes of data, we choose to represent our results using polar plots, as displayed in the case study below, to facilitate the comprehension of EEG readouts, and to allow our clients to get the most out of the analysis.
Polar Plots: a Glimpse into your Compound’s EEG Profile
In this case, we represented the dose-dependent effect of Haloperidol on the classical spectral bands, delta (2-4Hz), theta (4-8Hz), alpha (8-12Hz), beta (12-30Hz) and gamma (30-140Hz). Doses represented on a), b) & c) are 0.5mg/kg, 2mg/kg and 5mg/kg, respectively. We compared the doses in each 30-minute time window. The readout represents the absolute percent power change relative to baseline.
Polar plots are a powerful method to visualize the global pharmacodynamic profiles of compounds and their effects on frequency bands. They provide a compact representation of complex data with an intuitive circular layout, offering insights into pharmacokinetic and pharmacodynamic properties over time. To support our work, we also represent our data using more classical representations. For example, quantitative measures of spectral power within distinct frequency bands allow precise comparisons between baseline and post-administration EEG recordings and facilitate the identification of drug-induced changes.
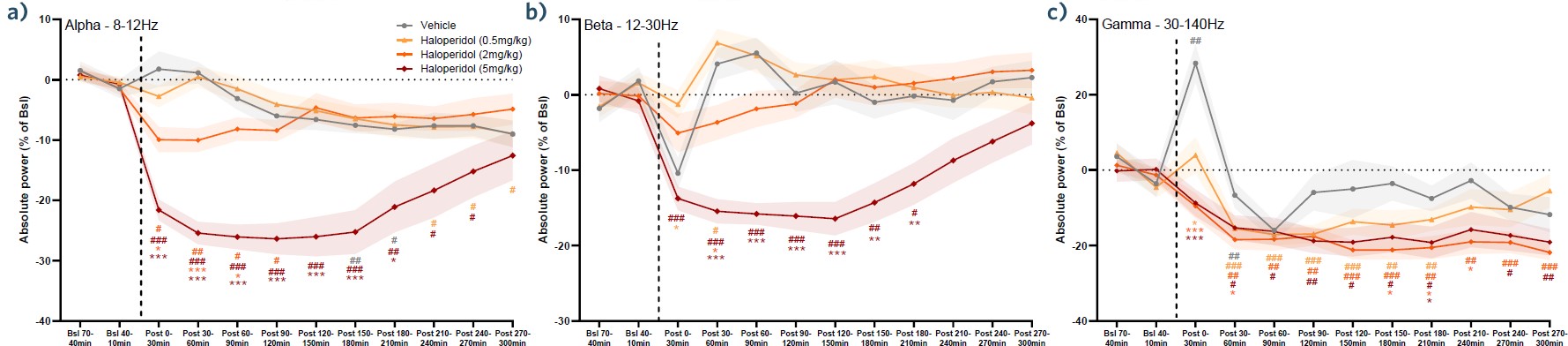
Fast Fourier Transform: In-depth Analysis of a Specific Band
This figure shows the time-course of the EEG power measured in the left prefrontal cortex, in the alpha (a), beta (b), and gamma (c) ranges. In addition to the vehicle condition (gray), data following administration of three doses of haloperidol are shown: 0.5mg/kg (light orange), 2mg/kg (dark orange), and 5mg/kg (red).
FFTs provide a different level of details to the polar plots. Although this type of analysis is quite straightforward, it represents an important step in compound characterization, providing information on the PK/PD as well as the power change of each of the spectral bands.
Coming Soon: Pharmaco-EEG for Coherence Analysis
SynapCell is constantly striving to push the boundaries of science and expand its innovation pipeline. For example, our R&D team is undertaking exploratory studies on coherence. Coherence is an endpoint that can be used to study how different structures communicate with each other. This can be perturbed, in particular, by compounds such as antipsychotics and antidepressants. It is therefore a relevant marker for most Pharmaco-EEG studies.
Want to know to what extent your compound changes the EEG signal between structures and how it impacts their communication?
Contact us!
Let's Talk About Your Research Project!
More than a CRO, a team of collaborators – we are your dream neuroscience team specialized in preclinical EEG! We don’t just produce data, we are your partners from conceptualization to conclusion. We translate raw EEG data into meaningful, clinically-relevant endpoints, delivering clear insights to allow data-based decision-making. Choose SynapCell, a leading preclinical CNS-specialized CRO for cutting-edge EEG expertise combined with an irresistible touch of fun.
News & Events
PRESS RELEASE
SynapCell and the University of Utah Celebrate the 10-year Anniversary of their Collaboration on Anti-Seizure Medications.
NEW!
AMYGDALA KINDLING MODEL
Choose our Amygdala Kindling model to test compounds targeting focal-to-bilateral tonic-clonic seizures. Choosing the right model for the appropriate type of epilepsy seizures is key to the effective discovery of ASMs.
NEW!
SLEEP & VIGILANCE STATES
Discover SynapCell’s new preclinical EEG capabilities for sleep and vigilance states, and gain additional insights to characterize compound effects.