GENERALIZED
ABSENCE EPILEPSY
Home » Solutions / Drug Efficacy Testing / Epileptic Disorders » Generalized Absence Epilepsy
Accelerating the Search for New Treatments for Generalized Absence Epilepsy
Generalized Absence Epilepsy is a common pediatric Epilepsy syndrome*. Involving the cortico-thalamic-cortical circuit, it is characterized by brief, non-convulsive seizures with behavioral arrest and generalized non-motor seizures. Patients experience short “blanking out” periods with immediate recovery. During these typical absence seizures, brain activity shows bilateral, synchronous spike-and-wave discharges (SWD).
Drug development for this form of Epilepsy faces major challenges, due to the limited efficacy of current treatments (mainly valproate and ethosuccimide) and their side effects. New approaches are therefore needed. Translational approaches must be redesigned to better integrate preclinical and clinical observations, and thus overcome difficulties in converting promising results into effective treatments. A paradigm shift in drug discovery is required, based on improved understanding of the mechanisms of epileptogenesis.
With this in mind, SynapCell offers the GAERS model and its related EEG biomarker, combined they considerably increase the predictability, from the pre-clinical stage, of molecule efficacy and effectiveness in humans.
*GAE affects 6-8/100,000 children up to 15 years of age versus a prevalence of 0.7 to 4.6/100,000 in the general population – Guilhoto 2016, Seizure.
GAERS Model & SynapCell’s EEG Biomarker: Translational Breakthroughs in Discovery of Antiseizure Medications
The Genetic Absence Epilepsy Rat from Strasbourg (GAERS) is a selectively inbred strain of Wistar rats displaying spontaneous spike-and-wave discharges (SWD). The GAERS model has been a reference for absence Epilepsy for more than 30 years. These rats present behavioral, electrophysiological and pharmacological features mirroring human absence seizures.
The GAERS model with its in vivo EEG SWD biomarker, developed by SynapCell, accurately mirrors human Epilepsy, offering robust endpoints for evaluation of compounds with the potential to treat generalized absence Epilepsy. The results reliably predict clinical outcomes.
The GAERS Model, the Reference in Absence Epilepsy
- Predictive of in-human effects
- Offers a pharmacosensitive EEG Biomarker (SWD)
- Derisks further Phase II studies
- Unveils potential seizure aggravating effects
- Predicts adverse effects of other drugs targeting the CNS (anti-psychotic and anxiolytic drugs)

A Predictive Approach with Translational EEG Biomarkers
Revolutionizing Epilepsy research, the GAERS model replicates human epilepsy patterns. By harnessing sophisticated EEG biomarkers (SWD, Spike-Wave Discharges), it is possible to track non-convulsive seizures. This groundbreaking technique, born from rigorous R&D, empowers drug developers by providing vital data on molecule effectiveness during preclinical phases. The result? A turbo-charged path to new treatments for Generalized Absence Epilepsy.
SynapCell's GAERS Model, a World-Exclusivity!
SynapCell holds the world-exclusive license for the GAERS model since 2007 and provides drug developers with a program dedicated to Epilepsy (ASM programs) capable of predicting in-human efficacy of antiseizure medications from the preclinical steps. It can be used to derisk the potential seizure-aggravating effects of any drug tested.
The GAERS model complements SynapCell’s established leadership in the Epilepsy market, alongside our proprietary Mesial Temporal Lobe Epilepsy (MTLE) mouse model and the Amygdala Kindling rat model.
“The GAERS model, which is a proven, early, informative indicator of efficacy in anti-seizure drug development with high predictability of response in humans, mimics behavioral, electrophysiological and pharmacological features of human absence seizures.”
Avenue Therapeutics
Key Features
of the GAERS Rat Model
The GAERS non-convulsive model mimics the behavioral, electrophysiological and pharmacological features clinically observed in human absence seizures. It is thus invaluable for drug discovery.
The GAERS is a non-convulsive rat model of generalized absence Epilepsy.
The GAERS model offers several key advantages for Epilepsy research:
- High predictive validity
- Robust and reproducible
- Pharmacologically relevant
- Mechanistic insights, paving the way for in-depth study of the neurobiological mechanisms underlying absence seizures and epileptogenesis
- Translational value
These advantages make the GAERS model a valuable tool for drug discovery in generalized absence epilepsy, offering a high degree of predictability and relevance to human conditions.
The spike-wave discharges (SWDs) observed in the GAERS model are easily quantifiable, providing robust and reproducible endpoints for compound evaluation.
The GAERS model displays spontaneous SWDs that closely mirror human absence seizures. This feature has made it the reference model for translational research into absence epilepsy for over 30 years.
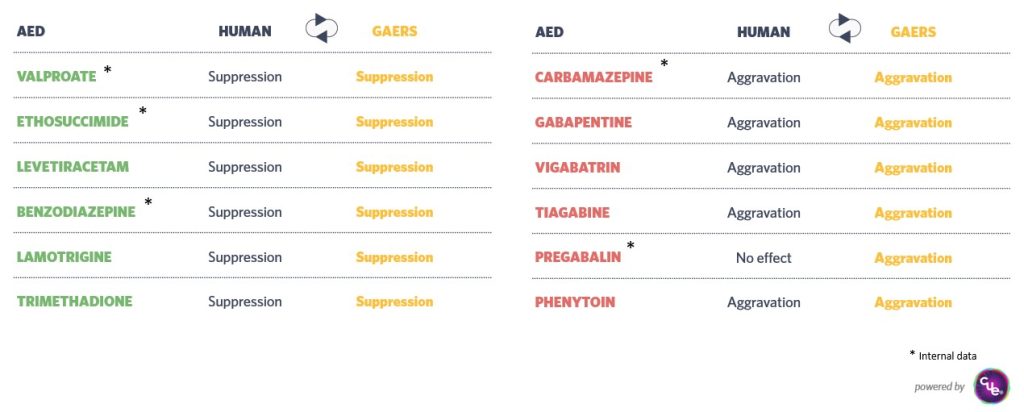
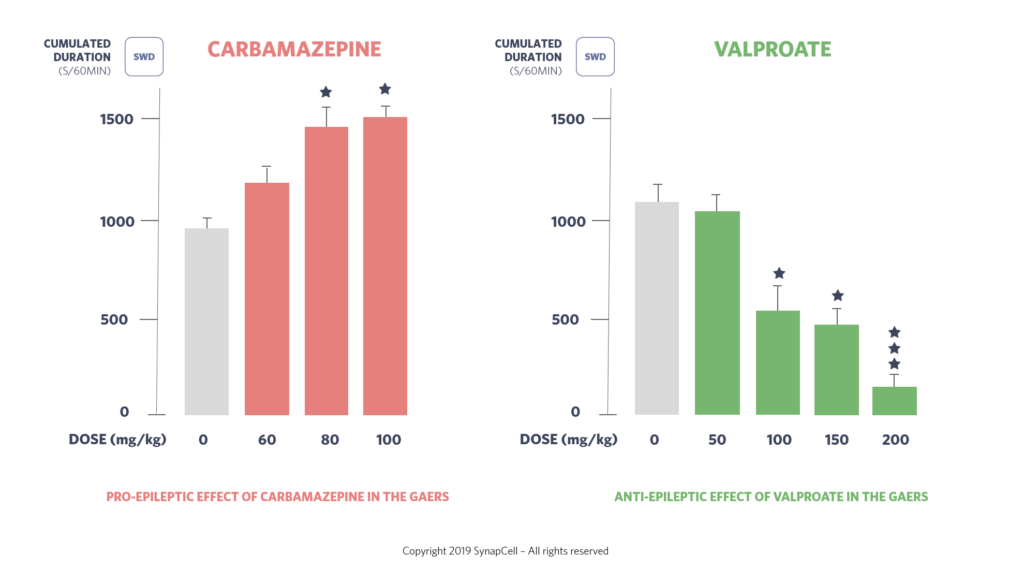
The GAERS model shows responses similar to humans when treated with anti-epileptic compounds such as valproate, ethosuximide, levetiracetam, benzodiazepine, lamotrigine, trimethadione, carbamazepine, gabapentin, vigabatrin, tiagabine, pregabalin, phenytoin. This similarity enhances its relevance for preclinical studies.
Drug Discovery Assays with the GAERS Rat Model
Identify, optimize and test promising compounds for efficacy and pharmacological properties to determine their potential as drug candidates.
Test two or more drugs in parallel to evaluate their relative efficacy and safety.
Evaluate drug interactions affecting efficacy or pharmacokinetics when used in combination. Identify potential risks or benefits of drug combinations early in development.
- Validate and confirm the antiseizure potential of a drug
- Evaluate the disease modifying potential of a drug
- Evaluate the potential loss of effect
Joint Publication SynapCell x Pfizer

POSTER
Pronounced Antiepileptic Activity of the Subtype-Selective GABAA Positive Allosteric Modulator PF-06372865 in the GAERS Model
The aim of this study was to assess the antiepileptic effect of PF-06372865, a newly developed subtype-selective GABAA positive allosteric modulator, developed by Pfizer, in a preclinical model of absence seizures, the GAERS rat. PF-06372865 dose-dependently reduced the occurrence of SWD in the GAERS model. The pharmacology of the GAERS model translates to the clinic. In comparison to other benzodiazepines, PF-06372865, is structurally different and has a better affinity for the GABAA receptor, with a less adverse effect.
Publication also available on PubMed.
DOWNLOAD
POSTER

Powered by Cue®, SynapCell's Predictive In Vivo EEG Platform
The GAERS rat model, for which SynapCell has worldwide exclusivity, and its associated EEG biomarker (SWD) are processed on Cue®, our innovative translational in vivo EEG platform, which is designed to predict the in-human efficacy of your drug candidates during the preclinical step. Cue® is the result of decades of R&D, combining SynapCell’s know-how, expertise and scientific excellence in the fields of brain surgery and EEG signal recording, processing, and analysis.
Using Cue®, we transform preclinical data into actionable insights, offering end-to-end support for informed decision-making in CNS drug discovery.
THE SCIENCE CORNER
The GAERS Pharmacology
Translates into the Clinic
Augment the predictability score of your compound with a powerful, robust and objective EEG biomarker of Absence Epilepsy.
More importantly, derisk any of your compounds screening for potential pro-epileptic effects and compare them to standards of care.
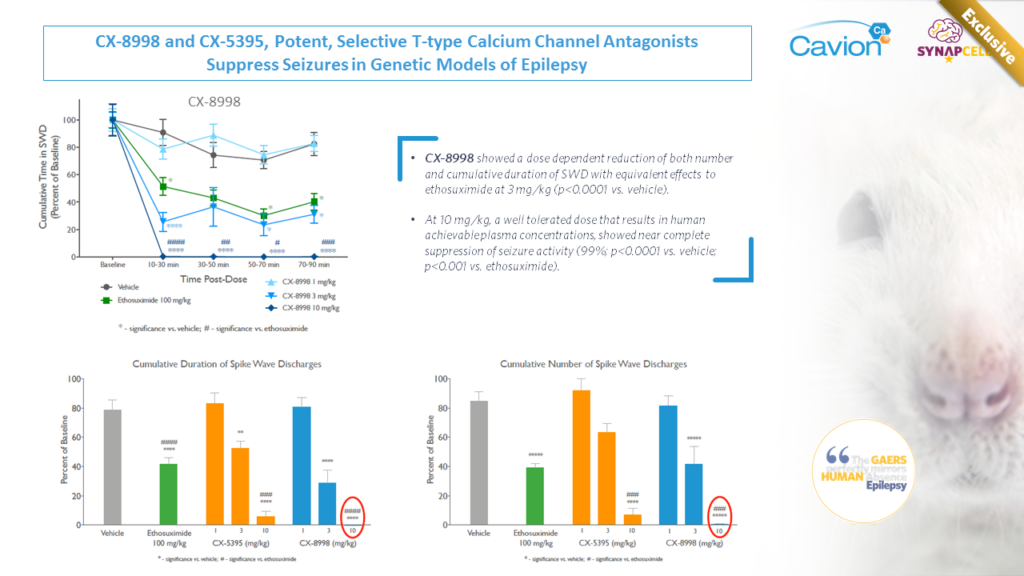
Joint Work with Cavion

Cavion’s T-Type Calcium Channel Modulator CX-8998 demonstrated a superior effect to the current standard of care in suppressing absence seizures in SynapCell’s Genetic Absence Epilepsy Model (GAERS).
Collaboration with Avenue Therapeutics
BAER-101, a selective potentiator of α2- and α3-containing GABAA receptors, fully suppresses spontaneous cortical spike-wave discharges in Genetic Absence Epilepsy Rats from Strasbourg (GAERS).
BAER-101

Let's Talk About Your Research Project!
More than a CRO, a team of collaborators – we are your dream neuroscience team specialized in preclinical EEG! We don’t just produce data, we are your partners from conceptualization to conclusion. We translate raw EEG data into meaningful, clinically-relevant endpoints, delivering clear insights to allow data-based decision-making. Choose SynapCell, a leading preclinical CNS-specialized CRO for cutting-edge EEG expertise combined with an irresistible touch of fun.
News & Events
PRESS RELEASE
SynapCell and the University of Utah Celebrate the 10-year Anniversary of their Collaboration on Anti-Seizure Medications.
NEW!
AMYGDALA KINDLING MODEL
Choose our Amygdala Kindling model to test compounds targeting focal-to-bilateral tonic-clonic seizures. Choosing the right model for the appropriate type of epilepsy seizures is key to the effective discovery of ASMs.
NEW!
SLEEP & VIGILANCE STATES
Discover SynapCell’s new preclinical EEG capabilities for sleep and vigilance states, and gain additional insights to characterize compound effects.